接上文Part.1
The Path and the Role of Copper [Cu] in Organisms/铜【Cu】在生物体中的路径和作用
Biological role of Cu/铜的生物学作用
Copper plays an important role in our metabolism, largely because it allows many critical enzymes to function properly [44]. Copper is essential for maintaining the strength of the skin, blood vessels, epithelial and connective tissue throughout the body. Cu plays a rolein the production of hemoglobin, myelin, melanin and it also keeps thyroid gland functioning normally [7,44,45]. Copper can act as both an antioxidant and a pro-oxidant. Free radicals occur naturally in the body and can damage cell walls, interact with genetic material, and contribute to the development of a number of health problems and diseases. As an antioxidant, Cu scavenges or neutralize free radicals and may reduce or help prevent some of the damage they cause [5,46-48]. When copper acts as a pro-oxidant at times, it promotes free radical damage and may contribute to the development of Alzheimer’s disease [49,50]. Maintaining the proper dietary balance of Cu, along with other minerals such as zinc and manganese, is important [5].
铜在我们的新陈代谢中扮演着重要角色,主要是因为它允许许多关键酶发挥正常功能 [44]。铜对于维持全身皮肤、血管、上皮和结缔组织的强度至关重要。铜在血红蛋白、髓磷脂、黑色素的产生中发挥作用,它还能保持甲状腺正常运作 [7,44,45]。铜可以作为抗氧化剂和促氧化剂。自由基在体内自然发生,会破坏细胞壁,与遗传物质相互作用,并导致许多健康问题和疾病的发展。作为一种抗氧化剂,铜可以清除或中和自由基,并可以减少或帮助阻止它们造成的一些伤害 [5,46-48]。当铜有时充当促氧化剂时,它会促进自由基损伤,并可能导致阿尔茨海默病的发展 [49,50]。保持铜以及锌和锰等其他矿物质的适当膳食平衡很重要 [5]。
Cu metabolism/铜代谢
Copper is absorbed in the gut and transported to the liver bound to albumin. It enters the bloodstream via the plasma protein called ceruloplasmin, where its metabolism is controlled, and is excreted in bile [51]. A transporter protein on the cells of the small bowel, copper membrane transporter 1 - CMT1, carries copper inside the cells, where some is bound to metallothioneins and part is carried by copper transport protein - ATOX1 (copper chaperone for ATP7A and ATP7B) to the trans-Golgi network. Here, in response to rising concentrations of copper, an enzyme called ATP7A releases copper into the portal vein to the liver. Liver cells also carry the CMT1 protein, and metallothioneins and ATOX1 bind Cu inside the liver cells, but here it is ATP7B that links copper to ceruloplasmin and releases it into the bloodstream, as well as removing excess copper by secreting it into bile [52]. Approximately 90% of the copper in the blood is incorporated into ceruloplasmin, which is responsible for carrying copper to tissues that need the mineral [44,45]. Since excretion of copper is so slow (10% in 72 hours) an excessive dose of Cu is a lingering problem [51]. Proper absorption and metabolism of copper requires an appropriate balance with the minerals zinc and manganese. Because zinc can compete withcopper in the small intestine and interfere with its absorption, persons who supplement with inappropriately high levels of zinc and lower levels of copper may increase their risk of copper deficiency [44,45]. In addition to the role of ceruloplasmin as a transport protein, it also acts as an enzyme; catalyzing the oxidation of minerals, most notably iron [51]. The oxidation of iron by ceruloplasmin is necessary for iron to be bound to its transport protein- transferrin, so iron deficiency anemias may be a symptom of copper deficiency [5,45]. Mutations in copper transport proteins, ATP7B and ATP7A, can disable these transport systems and leading to Wilson’s disease and Menke’s disease,respectively [53,54].
铜被肠道吸收并与白蛋白结合转运到肝脏。它通过称为铜蓝蛋白的血浆蛋白进入血液,在那里它的代谢受到控制,并在胆汁中排泄[51]。小肠细胞上的一种转运蛋白,铜膜转运蛋白1-CMT1,在细胞内携带铜,其中一些与金属硫蛋白结合,部分由铜转运蛋白-ATOX1(ATP7A和ATP7B的铜伴侣)携带至跨高尔基网络。在这里,作为对铜浓度升高的反应,一种称为ATP7A的酶将铜释放到门静脉中并进入肝脏。肝细胞也携带CMT1蛋白,金属硫蛋白和ATOX1在肝细胞内与铜结合,但在这里是ATP7B将铜与铜蓝蛋白连接并将其释放到血液中,并通过将铜分泌到胆汁中来去除多余的铜 [52],血液中大约 90% 的铜被掺入铜蓝蛋白中,铜蓝蛋白负责将铜运送到需要矿物质的组织中 [44,45]。由于铜的排泄很慢(72 小时内10%),解决铜过量是一个非常缓慢的问题 [51]。铜的正常吸收和代谢需要与矿物质锌和锰保持适当的平衡。因为锌可以与铜在小肠中竞争并干扰其吸收,因此补充不当,高水平锌和低水平铜的人可能会增加缺铜的风险 [44,45]。铜蓝蛋白除了作为转运蛋白,还起到酶的作用;催化矿物质的氧化,最显著的是铁[51]。铜蓝蛋白对铁的氧化是铁与其转运蛋白-转铁蛋白结合所必需的,因此缺铁性贫血可能是缺铜的症状[5,45]。铜转运蛋白ATP7B和 ATP7A的突变可以使这些转运系统失调并分别导致Wilson’s病和Menke’s病 [53,54]。
Cu enzymes/铜酶
Copper proteins have diverse roles in biological electron transport and oxygen transportation, processes that exploit the easy interconversion of Cu(I) and Cu(II) [55]. In cytochrome c oxidase,which is required for aerobic respiration, copper and iron cooperate in the reduction of oxygen. Copper is also found in Cu/Zn superoxide dismutase, an enzyme that detoxify superoxides, by converting it to oxygen and hydrogen peroxide: 2 HO2 → H2O2 + O2 [7,55]. Copper is also a component of lysyl oxidase, an enzyme that participates in the synthesis of collagen and elastin, two important structural proteins found in bone and connective tissue. As a part of the enzymes cytochrome c oxidase copper plays a role in energy production, as part of dopamine ß-hydroxylase a role in conversion of dopamine to norepinephrine, and with Factor IV helps in blood clotting. Copper is also important for the production of the thyroid hormone thyroxine. A copper- containing enzyme tyrosinase, converts tyrosine to melanin. Cu is also necessary for the synthesis of phospholipids found in myelin sheaths in peripheral nerves [5,7,44,45]. Several Cu protein do not interact directly with substrates, hence they are not enzymes. These proteins relay electrons by the process called electron transfer [55].
铜蛋白在生物电子传递和氧气传递中发挥着不同的作用,这些过程利用了铜(I) 和铜(II) 的简单相互转化 [55]。在有氧呼吸所需的细胞色素C氧化酶中,铜和铁协同还原氧气。铜也存在于铜/锌超氧化物歧化酶中,这是一种通过将超氧化物转化为氧分子和过氧化氢来解毒的酶:2HO2→H2O2+O2 [7,55]。铜也是赖氨酰氧化酶的一种成分,这种酶参与胶原蛋白和弹性蛋白的合成,这两种重要的结构蛋白存在于骨骼和结缔组织中。作为酶的一部分,含铜细胞色素C氧化酶在能量产生中发挥作用,作为多巴胺ß-羟化酶的一部分,在多巴胺向去甲肾上腺素的转化中起作用,并且与因子IV一起有助于血液凝固。铜对甲状腺激素中甲状腺素的产生也很重要。一种含铜的酪氨酸酶,可将酪氨酸转化为黑色素。铜也是合成周围神经髓鞘中的磷脂所必需的 [5,7,44,45]。很多铜蛋白不直接与底物相互作用,因此它们不是酶。这些蛋白质通过称为电子转移的过程传递电子 [55]。
Cu/Zn Superoxide dismutase1 [Cu/ZnSOD1] / 铜/锌超氧化物歧化酶1【Cu/ZnSOD1】
Cu/ZnSOD1 is one of the three human superoxide dismutases identified and characterized in mammals (SOD2-manganese superoxide dismutase and SOD3-extracellular superoxide dismutase) [56,57,58]. Cu/ZnSOD1 is a homodimeric detoxifying metalloenzyme localized mostly in the cytosol and also in nucleus, peroxisomes, and mitochondria [56]. When SOD1 was isolated for the first time, it was thought to be a copper storage protein; the catalytic function of SOD1 was discovered in 1969 and it was clear that SOD1 acts as a scavenger of superoxide, through a two-step reaction involving reduction and reoxidation of the copper ion in its active site (Figure 3) [59]. Primarily, this reaction occurs in the cytoplasm where SOD1 is highly expressed. In the 1990s, the scientific community focused their studies on the genetic and biochemical characterization of SOD1, demonstrating that SOD1 is linked to diseases as inflammatory bowel disease [60], cancer [61], hypertension and cardiovascular diseases [62,63], Down’s syndrome [64] and amyotrophic lateral sclerosis [65,66,67]. In 1993, the first SOD1 gene mutations associated with ALS were described [56]. The human SOD1 gene is located on chromosome 21q22.11, and it codes for the monomeric SOD1 polypeptide (153 amino acids, molecular weight 16 kDa). The coding region consists of five exons interrupted by four introns. Several polymorphisms have been identified in SOD1 gene, mainly distributed in the regulatory regions, including promoter and introns [68].
Cu/ZnSOD1是在哺乳动物中鉴定和表达的三种人类超氧化物歧化酶之一(SOD2-锰超氧化物歧化酶和SOD3-细胞外超氧化物歧化酶)[56,57,58]。Cu/ZnSOD1是一种同二聚体解毒金属酶,主要位于胞质及细胞核、过氧化物酶体和线粒体中 [56]。首次分离SOD1时,认为它是一种铜贮藏蛋白;SOD1的催化功能是在1969年发现的,很明显SOD1作为超氧化物的清除剂,通过涉及其活性位点中铜离子的还原和再氧化的两步反应(图 3)[59]。这种反应主要发生在SOD1高表达的细胞质中。在1990年代,科学界将研究重点放在SOD1的遗传和生化特征上,证明SOD1与炎症性肠病 [60]、癌症 [61]、高血压和心血管疾病 [62,63]、唐氏综合征 [64] 和肌萎缩侧索硬化症 [65,66,67]。1993年,第一个与ALS相关的SOD1基因突变被描述 [56]。人类SOD1基因位于染色体21q22.11,编码单体SOD1多肽(153个氨基酸,分子量16 kDa)。编码区由被四个内含子中断的五个外显子组成。在SOD1基因中已鉴定出几种多态性,主要分布在调控区,包括启动子和内含子[68]。
Superoxide radicals are generated during normal metabolism, as well as when white blood cells attack invading bacteria and viruses (phagocytosis). If not eliminated quickly, superoxide radicals causedamage to cell membranes [5,48]. When copper is not present in sufficient quantities, the activity of superoxide dismutase is diminished, and the damage to cell membranes caused by superoxide radicals is increased. When functioning in this enzyme, copper works together with the mineral zinc, and it is actually the ratio of copper to zinc, rather than the absolute amount of copper or zinc alone, that helps the enzyme function properly (Figure 4) [44,45].
在正常新陈代谢过程中以及当白细胞攻击入侵的细菌和病毒(吞噬作用)时会产生超氧自由基。如果不迅速消除,超氧自由基会导致细胞膜损伤[5,48]。当铜含量不足时,超氧化物歧化酶的活性降低,超氧自由基对细胞膜的损伤增加。在这种酶中起作用时,铜与矿物质锌一起起作用,实际上是铜与锌的比例,而不是单独的铜或锌的绝对量,有助于酶正常发挥作用(图 4)[44,45 ]。
How coordination of metal ions modulates protein structures is not only important for elucidating biological function but has also emerged as a key determinant in protein turnover and protein-misfolding diseases [69]. The study of folding catalysis by transient coordination of Zn to the Cu ligands of the Cu/Zn superoxide dismutase showed that the coordination of Zn to the enzyme Cu/Zn superoxide dismutase is directly controlled by the protein’s folding pathway. Zn first catalyzes the folding reaction by coordinating transiently to the Cu ligands of SOD1. Then, after the global folding transition has commenced, the Zn2+ ion transfers to the higher affinity Zn site, which structures only very late in the process. The relatively rapid equilibration of metals in and out of the SOD1 structure [10-5s-1] provided an explanation: if a dissociated Zn2+ ion is prevented from rebinding to the SOD1 structure then the lifetime of the encyme is reduced to a just a few hours [69].
金属离子的配位如何调节蛋白质结构不仅对于阐明生物学功能很重要,而且已成为蛋白质周转和蛋白质错误折叠疾病的关键决定因素[69]。通过锌与铜/锌超氧化物歧化酶的铜配体的瞬时配位对折叠催化的研究表明,锌酶与铜/锌超氧化物歧化酶的配位直接受蛋白质折叠途径的控制。锌首先通过与SOD1的铜配体瞬时配位来催化折叠反应。然后,在全局折叠转变开始后,Zn2+离子转移到亲和力更高的锌位点,该位点仅在该过程的后期才形成。金属进出SOD1结构相对快速的平衡 [10-5s-1] 给出了一个解释:如果阻止解离的Zn2+离子重新结合到SOD1结构上,那么酶的寿命就会减少到只有几个小时 [69]。
The maturation and activation of the Cu/Zn superoxide dismutase (SOD1) are highly regulated processes that require several posttranslational modifications. The maturation of SOD1 is initiated by incorporation of zinc and copper ions followed by disulfide oxidation leading to the formation of enzymatically active homodimers [70,71]. Present data indicate that homodimer formation is a regulated final step in SOD1 maturation and implicate the recently characterized copper homeostasis protein COMMD1 in this process. COMMD1 interacts with SOD1, and this interaction requires copper incorporation into SOD1. COMMD1 does not regulate disulfide oxidation of SOD1 but reduces the level of enzymatically active SOD1 homodimers. RNAi mediated knockdown of COMMD1 expression results in a significant induction of SOD1 activity and a consequent decrease in superoxide anion concentrations, whereas overexpression of COMMD1 exerts exactly the opposite effects. COMMD1 is a novel protein regulating SOD1 activation and associate COMMD1 function with the production of free radicals [70,71].
铜/锌超氧化物歧化酶 (SOD1) 的成熟和活化是高度调控的过程,需要进行多次翻译后修饰。SOD1的成熟是由锌和铜离子的掺入引发的,然后是二硫化物氧化,导致形成具有酶活性的同型二聚体 [70,71]。目前的数据表明,同源二聚体的形成是SOD1 成熟过程中受调控的最后一步,并暗示在这个过程中表达的铜稳态蛋白COMMD1与SOD1相互作用需要将铜掺入到SOD1中。COMMD1不调节SOD1的二硫化物氧化,但会降低酶活性SOD1同源二聚体的水平。RNAi介导的COMMD1表达下调导致SOD1活性的显著诱导和随之而来的超氧阴离子浓度的降低,而COMMD1的过表达则产生完全相反的效果。COMMD1是一种调节SOD1活化并将COMMD1功能与自由基产生相关联的新型蛋白质 [70,71]。
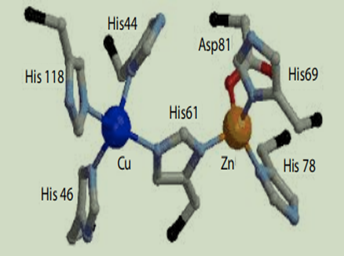
图 3:铜/锌超氧化物歧化酶1的活性位点
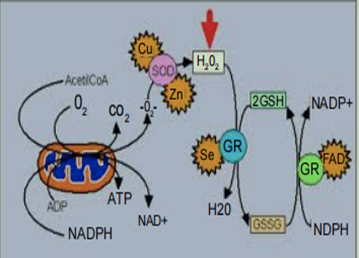
图 4:Cu/Zn SOD主要存在于细胞质中
Cu chaperones/铜伴侣
The intracellular trafficking of Cu to copper-dependent proteins is fundamental to normal cellular metabolism. The copper chaperones perform the dual functions of trafficking and the prevention of cytoplasmic exposure to copper ions in transit. Only a small number of copper chaperones have been identified at this time but their conservation across plant, bacterial and animal species suggests that the majority of living systems utilise these proteins for copper routing [72]. The available data suggest that each copper-dependent protein in the cell is served by a specific copper chaperone. Specificity for a given copper-dependent protein appears to be mediated by the residues surrounding the copper-binding motif [73].
铜向铜依赖性蛋白细胞内的运输是正常细胞代谢的基础。铜伴侣具有运输和防止细胞质在运输过程中暴露于铜离子的双重功能。目前仅鉴定出少量铜伴侣,但它们在植物、细菌和动物物种中的保护表明大多数生命系统利用这些蛋白质进行和铜相关的代谢 [72]。现有数据表明,细胞中的每种铜依赖蛋白都由特定的铜伴侣提供服务。对给定的铜依赖性蛋白的特异性似乎是由铜结合基序周围的残基介导的 [73]。
Copper is needed within mitochondria to supply the Cu proteins and intramembrane Cu sites of cytochrome oxidase, within the transGolgi network to supply secreted cuproproteins and within the cytosol to supply Cu/Zn superoxide dismutase1 (SOD1). Subpopulations of copper-zinc superoxide dismutase also localize to mitochondria, the secretory system and the nucleus [74]. Copper metallochaperones assist copper in reaching vital destinations without inflicting damage or becoming trapped in adventitious binding sites [72]. Copper ions are specifically released from copper metallochaperones upon contact with their cognate cuproproteins, so metal transfer is thought to proceed by ligand substitution [73].
线粒体内需要铜来供应铜蛋白和细胞色素氧化酶的膜内铜位点,跨高尔基网络内需要铜来供应分泌的铜蛋白,而细胞质内需要铜来供应铜/锌超氧化物歧化酶1(SOD1)。铜锌超氧化物歧化酶的亚群也定位于线粒体、分泌系统和细胞核 [74]。铜金属伴侣帮助铜到达重要目的地,而不会造成损害或被困在不定的结合位点 [72]。铜离子在与其同源的铜蛋白接触时从铜金属伴侣中特异性地释放出来,因此金属转移被认为是通过配体取代进行的 [73]。
Copper chaperone for SOD1 specifically delivers copper to SOD1 in cytoplasm of mammalian cells. In the present study, small interfering RNA targeting copper chaperone for SOD1 (CCS) was introduced into metallothionein-knockout mouse fibroblasts (MT-KO cells) and their wild type cells (MT-WT cells) to reveal the interactive role of CCS with other Cu-regulating proteins. CCS knockdown significantly decreased the level of Cu influx transporter (Ctr1). On the other hand, mRNA expression for a Cu efflux transporter (ATP7A) was increased and was 3.0 - 2.5 times higher than those of the control. The experiment also showed that the ATP7A knockdown significantly increased the intracellular Cu concentration. The activity of SOD1 were maintained in both MT-WT and MT-KO cells even when copper chaperone for SOD1 protein expression was reduced to 0.30-0.35 of control. This suggests that the amount of CCS protein exceeds that required to supply Cu to SOD1 in the cells. Further, the CCS knockdown induces Cu accumulation in cells. The results showed that Cu accumulation is ameliorated by the metallothionein induction, the decrease of Ctr1 expression and the increase of ATP7A expression to maintain Cuhomeostasis [76].
SOD1的铜分子伴侣特异性地将铜输送到哺乳动物细胞质中的SOD1。在本研究中,将靶向SOD1铜伴侣的小干扰RNA(CCS)引入敲除金属硫蛋白的小鼠成纤维细胞(MT-KO细胞)及其野生型细胞(MT-WT细胞)中以揭示CCS与其他细胞的相互作用。铜调节蛋白。CCS敲除显著降低了铜流入转运蛋白(Ctr1)的水平。另一方面,铜外排转运蛋白(ATP7A)的mRNA表达增加,比对照高3.0-2.5倍。实验还表明,ATP7A敲除显著增加了细胞内铜浓度。即使当用于SOD1蛋白表达的铜伴侣降低到对照的0.30-0.35时,SOD1的活性在MT-WT和MT-KO细胞中都保持不变。这表明CCS蛋白的量超过了向细胞中的SOD1提供铜所需的量。此外,CCS敲除诱导细胞中铜的积累。结果表明,金属硫蛋白的诱导、Ctr1表达的降低和ATP7A表达的增加可改善铜的积累,以维持铜的稳态[76]。
Sources of Copper and Zinc and Dietary Intakes/铜和锌的来源和膳食摄入量
Food sources/食物来源
A daily intakes of Cu and Zn is required to maintain a steady state because the body has no specialized storage system of these two elements [5,12,77]. A wide variety of foods contain zinc [18]. Oysters contain more zinc per serving than any other food, but red meat, especially beef, lamb and liver have some of the highest concentrations of zinc in food [78]. Other good food sources include beans, nuts, other types of seafood (such as crab and lobster), whole grains, cereals, almonds, pumpkin seeds, sunflower seeds [18,78]. Rich sources of copper include oysters and other sea food, beef and organ meats (especially liver), dark green leafy vegetables, enriched cereals, nutsand sunflower seeds, green olives, avocados, dried legumes, chocolate, cocoa, and black pepper [5,45].
需要每天摄入铜和锌以保持稳定状态,因为人体没有这两种元素的专门储存系统 [5,12,77]。各种各样的食物都含有锌 [18]。牡蛎每份的锌含量比任何其他食物都多,但红肉,尤其是牛肉、羊肉和肝脏,是食物中锌含量最高的部分 [78]。其他良好的食物来源包括豆类、坚果、其他类型的海鲜(如螃蟹和龙虾)、全谷物、谷物、杏仁、南瓜子、葵花子 [18,78]。丰富的铜来源包括牡蛎和其他海鲜、牛肉和内脏肉(尤其是肝脏)、深绿色叶菜、浓缩谷物、坚果和葵花籽、绿橄榄、鳄梨、干豆类、巧克力、可可和黑胡椒 [5 ,45]。
Dietary Supplements/膳食补充剂
Supplements contain several forms of zinc, including zinc gluconate, zinc sulfate, and zinc acetate. The percentage of elemental zinc varies by form of supplement [79].Scientists has not determined whether differences exist among forms of zinc in absorption, bioavailability, or tolerability [17]. Multivitamins that include minerals generally provide copper. It is also available as an individual oral supplement. Cu supplements should not be given to children, at children it should be obtained from food [5,7].
补充剂含有多种形式的锌,包括葡萄糖酸锌、硫酸锌和乙酸锌。元素锌的百分比因补充剂的形式而异 [79]。科学家尚未确定锌的吸收、生物利用度或耐受性是否存在差异 [17]。包含矿物质的多种维生素通常提供铜。它也可作为单独的口服补充剂使用。铜补充剂不应给予儿童,儿童应从食物中获得 [5,7]。
Recommended intakes for Zn/锌的推荐摄入量
Intake recommendations for Zn are provided in the Dietary Reference Intakes developed by the Food and Nutrition Board (FNB) at the Institute of Medicine of the National Academies. The current Recommended Dietary Allowances [RDA] for zinc is listed in Table 1 [18]. For infants aged 0-6 months, the FNB established an Adequate Intake * for Zn (= intake of Zn in breastfed infants) [80,81]. U.S. National Research Council set a Tolerable Upper Intake for adults of 40 mg/day [82,83].
美国国家科学院医学研究所食品和营养委员会(FNB)制定的膳食参考摄入量中提供了锌的摄入量建议。目前锌的推荐膳食摄入量[RDA]列于表1 [18]。对于0-6个月的婴儿,FNB确定了锌的充足摄入量*(=母乳喂养婴儿的锌摄入量)[80,81]。美国国家研究委员会将成人的可耐受上限摄入量设定为40毫克/天 [82,83]。
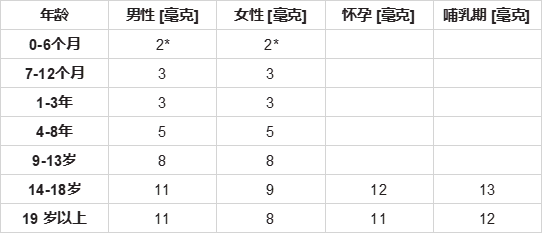
表 1:每日锌的推荐膳食摄入量 [RDAs]
Recommended intakes for Cu/铜的推荐摄入量
The 10th edition of Recommended Dietary Allowances (RDA) did not include an RDA for copper; rather a safe and adequate daily intake was suggested. Some nutritionists think, committees that establish RDAs should return to the traditions of the first nine editions and make recommendations that meet functional needs, because lack of a recommended dietary allowance for copper may be hazardous to health [81,84]. The following Table 2 provide the Recommended daily dietary intake (RDI) of copper for children and adults and Tolerable upper intake levels for copper [83,85].
第10版的推荐膳食津贴(RDA)不包括铜的RDA,而是建议安全和充足的每日摄入量。一些营养学家认为,建立RDA的委员会应该回到前九版的传统,并提出满足功能需求的建议,因为缺乏推荐的铜膳食摄入量可能对健康有害 [81,84]。下表2提供了儿童和成人铜的推荐每日膳食摄入量(RDI)和铜的可耐受上限 [83,85]。
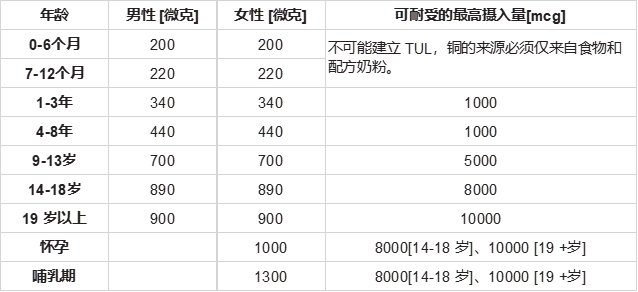
表 2:铜的推荐每日摄入量 [RDI] 和可耐受摄入量 [TULs]
接Part.3
- 还没有人评论,欢迎说说您的想法!
